Author: JianfuChen
Heterogeneous Catalysis
With the aid of first-principles calculations and our developed microkinetic tool (CATKINAS), we have explored many surface reactions, including CO oxidation, water-gas shift reaction, NO oxidation, NH3-SCR, HDO reaction and so on, which are promoted by different heterogenesous catalysts (e.g. metal, trantstion metal oxide, rare-earth oxide, zeolite, MX (X=C, N, S et al)). For instance, an interesting finding is that water deactivates metal oxides to CO oxidation and promotes low‐temperature CO oxidation with metals. Recently, we have been focusing on elucidating the dynamic evolution mechanism of catalytic systems with quantum chemistry method.
Importantly, we are making great effort to develop new catalyst screening methods and advanced catalysis theory, which are within or beyond the traditional volcano-curve framework. As a representative result, we proposed the half principle which can enable us to locate the efficient catalyst directly in many systems.
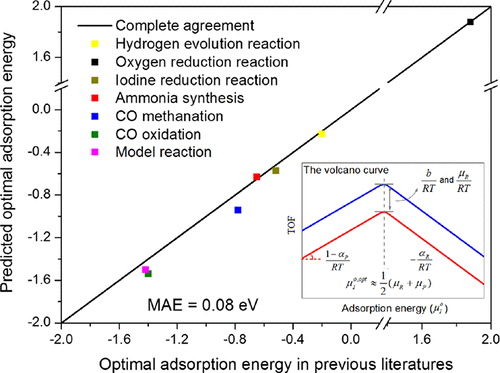
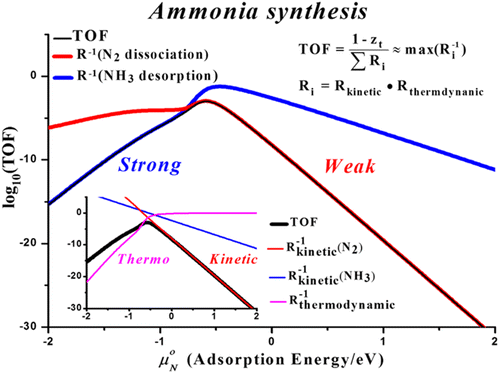
In addition, in order to explain the interesting phenomena in catalysis at the atomic level, we cooperated with experiment chemists, and thus more in-depth insights on the microscopic nature of catalyst have been achieved.
Publications
66. JF Chen; ML Jia; PJ Hu; HF Wang, CATKINAS: A large-scale catalytic microkinetic analysis software for mechanism auto-analysis and catalyst screening. J Comput Chem. 2021, 42, 379-391.
65. WB Xie; JY Xu; JF Chen; HF Wang; P Hu, Achieving Theory-Experiment Parity for Activity and Selectivity in Heterogeneous Catalysis Using Microkinetic Modeling. Acc. Chem. Res. 2022.
64. JF Chen; ML Jia; JL Wang; PJ Hu; HF Wang, Breaking through the Peak Height Limit of the Volcano-Shaped Activity Curve for Metal Catalysts: Role of Distinct Surface Structures on Transition Metal Oxides. J. Phys. Chem. C 2022, 126 (1), 183-191.
63. ZZ Lai; JF Chen; ML Jia; PJ Hu; HF Wang, Universal Skeleton Feature of the Three-Dimensional Volcano Surface and the Thermodynamic Rule in Locating the Catalyst in Heterogeneous Catalysis. ACS Catal. 2022, 12 (1), 247-258.
62. C Zhou; P Hu; HF Wang, Resolving the Two-Track Scaling Trend for Adsorbates on Rutile-Type Metal Oxides: New Descriptors for Adsorption Energies. J. Phys. Chem. C 2021, 125 (42), 23162-23168.
61. ZZ Lai; NL Sun; JM Jin; JF Chen; HF Wang; P Hu, Resolving the Intricate Mechanism and Selectivity of Syngas Conversion on Reduced ZnCr2Ox: A Quantitative Study from DFT and Microkinetic Simulations. ACS Catal. 2021, 11 (21), 12977-12988.
60. GT Chai; SN Pan; YL Guo; WC Zhan; L Wang; Y Guo; HF Wang, Insight into the Surface-Tuned Activity and Cl2/HCl Selectivity in the Catalytic Oxidation of Vinyl Chloride over Co3O4(110) versus (001): A DFT Study. J. Phys. Chem. C 2021, 125 (31), 16975-16983.
59. BB Xu; M Zhou; M Ye; LY Yang; HF Wang; XL Wang; YF Yao, Cooperative Motion in Water-Methanol Clusters Controls the Reaction Rates of Heterogeneous Photocatalytic Reactions. J. Am. Chem. Soc. 2021, 143 (29), 10940-10947.
58. MY Chen; J Xia; H Li; XG Zhao; QP Peng; JJ Wang; HH Gong; S Dai; PF An; HF Wang; ZS Hou, A Cationic Ru(II) Complex Intercalated into Zirconium Phosphate Layers Catalyzes Selective Hydrogenation via Heterolytic Hydrogen Activation. ChemCatChem 2021, 13, 3801-3814.
57. BH Zhang; JF Chen; GS Wu; Y Guo; HF Wang, Revealing the boosting role of NO for soot combustion over CeO2(111): A first-principles microkinetic modeling. Molecular Catalysis 2021, 509, 111582.
56. HY Yuan; HG Yang; P Hu; HF Wang, Origin of Water-Induced Deactivation of MnO2-Based Catalyst for Room-Temperature NO Oxidation: A First-Principles Microkinetic Study. ACS Catal. 2021, 11 (12), 6835-6845.
55. C Peng; JF Chen; PJ Hu; HF Wang, Molecular Adsorption Kinetics: Nonlinear Entropy-Enthalpy Loss Quantified by Constrained AIMD and Insights into the Adsorption-Site Determination on Metal Oxides. J. Phys. Chem. C 2021, 125(20), 10974-10982.
54. YX Jing; YQ Wang; SY Furukawa; J Xia; CY Sun; MJ Hulsey; HF Wang; Y Guo; XH Liu; N Yan, Towards the Circular Economy: Converting Aromatic Plastic Waste Back to Arenes over a Ru/Nb2O5 Catalyst. Angew.Chem.Int.Ed. 2021, 60 (10),5527-5535.
53. ZD Zhang; M Zhou; YJ Chen; SJ Liu; HF Wang; J Zhang; SF Ji; DS Wang; YD Li, Pd single-atom monolithic catalyst: Functional 3D structure and unique chemical selectivity in hydrogenation reaction. Sci China Mater 2021, 64 (8), 1919-1929.
52. JF Chen; ML Jia; ZZ Lai; PJ Hu; HF Wang, SSIA: A sensitivity-supervised interlock algorithm for high-performance microkinetic solving. J. Chem. Phys. 2021, 154 (2), 024108.
51. C Zhou; JY Zhao; PF Liu; JF Chen; S Dai; HG Yang; P Hu; HF Wang, Towards the object-oriented design of active hydrogen evolution catalysts on single-atom alloys. Chem. Sci. 2021, 12 (31), 10634-10642.
50. L Dong; J Xia; Y Guo; XH Liu; HF Wang; YQ Wang, Mechanisms of Caromatic-C bonds cleavage in lignin over NbOx-supported Ru catalyst. Journal of Catalysis 2021, 394, 94-103.
49. HH Gong; C Zhou; Y Cui; S Dai; XG Zhao; RH Luo; PF An; H Li; HF Wang; ZS Hou, Direct Transformation of Glycerol to Propanal using Zirconium Phosphate-Supported Bimetallic Catalysts. ChemSusChem 2020, 13(18),4954-4966.
48. C Zhou; HY Yuan; P Hu; HF Wang, A general doping rule: rational design of Ir-doped catalysts for the oxygen evolution reaction. Chem. Commun. 2020, 56(96), 15201-15204.
47. C Wang; HY Yuan; GZ Lu; HF Wang, Oxygen vacancies and alkaline metal boost CeO2 catalyst for enhanced soot combustion activity: A first-principles evidence. Applied Catalysis B: Environmental 2020, 119468.
46. XX Ji; HF Wang; P Hu, First principles study of Fenton reaction catalyzed by FeOCl: reaction mechanism and location of active site. Rare Metals 2019, 38 (8), 783-792.
45. JM Jin; JF Chen; HF Wang; P Hu, Insight into room-temperature catalytic oxidation of NO by CrO2 (110): A DFT study. Chinese Chemical Letters 2019, 30 (3), 618-623.
44. JF Chen; Y Mao; HF Wang; P Hu, A Simple Method To Locate the Optimal Adsorption Energy for the Best Catalysts Directly. ACS Catalysis 2019, 9 (3), 2633-2638.
43. CX Guo; ZY Wang; D Wang; HF Wang; P Hu, First-principles determination of CO adsorption and desorption on Pt(111) in the free energy landscape. The Journal of Physical Chemistry C 2018, 122 (37), 21478-21483.
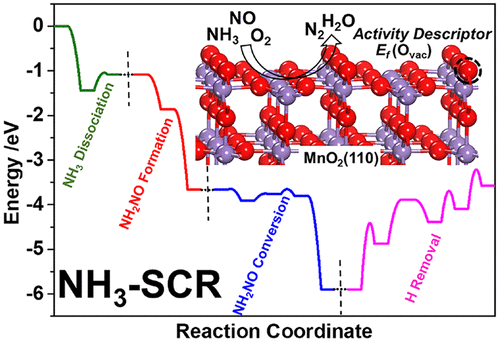
42. HY Yuan; NN Sun; JF Chen; JM Jin; HF Wang; P Hu, Insight into the NH3-Assisted Selective Catalytic Reduction of NO on β-MnO2(110): Reaction Mechanism, Activity Descriptor, and Evolution from a Pristine State to a Steady State. ACS Catalysis 2018, 8 (10), 9269-9279.
41. JM Jin; NL Sun; WD Hu; HY Yuan; HF Wang; P Hu, Insight into Room-Temperature Catalytic Oxidation of Nitric oxide by Cr2O3: A DFT Study. ACS Catalysis 2018, 8 (6), 5415-5424.
40. HY Yuan; JF Chen; YL Guo; HF Wang; P Hu, Insight into the Superior Catalytic Activity of MnO2 for Low-Content NO Oxidation at Room Temperature. The Journal of Physical Chemistry C 2018, 122 (44), 25365-25373.
39. HY Yuan; JF Chen; HF Wang; P Hu, Activity trend for low-concentration NO oxidation at room temperature on rutile-type metal oxides. ACS Catalysis 2018, 8 (11), 10864-10870.
38. JL Wang; HF Wang; P Hu, Theoretical insight into methanol steam reforming on indium oxide with different coordination environments. Science China Chemistry 2018, 61 (3), 336-343.
37. WB Ma; HY Yuan; HF Wang; QQ Zhou; K Kong; DF Li; YY Yao; ZS Hou, Identifying catalytically active mononuclear peroxoniobate anion of ionic liquids in the epoxidation of olefins. ACS Catalysis 2018, 8 (5), 4645-4659.
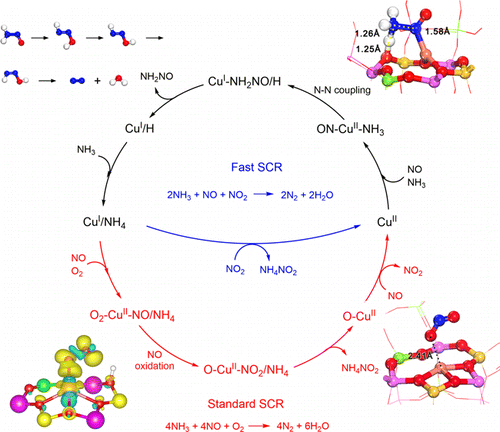
36. MX Yang; HY Yuan; HF Wang; P Hu, Insights into the selective catalytic reduction of NO by NH3 over Mn3O4(110): a DFT study coupled with microkinetic analysis. Science China Chemistry 2018, 61 (4), 457-467.
35. GN Li; L Li; HY Yuan; HF Wang; HR Zeng; JL Shi, Alkali-assisted mild aqueous exfoliation for single-layered and structure-preserved graphitic carbon nitride nanosheets. Journal of Colloid and Interface Science 2017, 495, 19-26.
34. W Ma; H Ma; JF Chen; YY Peng; ZY Yang; HF Wang; YL Ying; H Tian; YT Long, Tracking motion trajectories of individual nanoparticles using time-resolved current traces. Chemical science 2017, 8 (3), 1854-1861.
33. WC Zhan; JL Wang; HF Wang; JS Zhang; XF Liu; PF Zhang; MF Chi; YL Guo; Y Guo; GZ Lu, Crystal structural effect of AuCu alloy nanoparticles on catalytic CO oxidation. Journal of the American Chemical Society 2017, 139 (26), 8846-8854.
32. Y Mao; HF Wang; P Hu, Theory and applications of surface micro‐kinetics in the rational design of catalysts using density functional theory calculations. Wiley Interdisciplinary Reviews: Computational Molecular Science 2017, 7 (6), e1321.
31. ZJ Chen; Y Mao; JF Chen; HF Wang; YD Li; P Hu, Understanding the dual active sites of the FeO/Pt(111) interface and reaction kinetics: density functional theory study on methanol oxidation to formaldehyde. ACS Catalysis 2017, 7 (7), 4281-4290.
30. YM Dai; ZJ Chen; YL Guo; GZ Lu; YF Zhao; HF Wang; P Hu, Significant enhancement of the selectivity of propylene epoxidation for propylene oxide: a molecular oxygen mechanism. Physical Chemistry Chemical Physics 2017, 19 (36), 25129-25139.
29. HF Wang; D Wang; X Liu; YL Guo; GZ Lu; P Hu, Unexpected C-C bond cleavage mechanism in ethylene combustion at low temperature: origin and implications. ACS Catalysis 2016, 6 (8), 5393-5398.
28. QN Xia; ZJ Chen; Y Shao; XQ Gong; HF Wang; XH Liu; SF Parker; X Han; SH Yang; YQ Wang, Direct hydrodeoxygenation of raw woody biomass into liquid alkanes. Nature communications 2016, 7, 11162.
27. Y Mao; Z Wang; HF Wang; P Hu, Understanding Catalytic Reactions over Zeolites: A Density Functional Theory Study of Selective Catalytic Reduction of NOx by NH3 over Cu-SAPO-34. ACS Catalysis 2016, 6 (11), 7882-7891
26. D Wang; J Jiang; HF Wang; P Hu, Revealing the volcano-shaped activity trend of triiodide reduction reaction: a DFT study coupled with microkinetic analysis. ACS Catalysis 2016, 6 (2), 733-741.
25. C Chen; HY Yuan; HF Wang; YF Yao; WB Ma; JZ Chen; ZS Hou, Highly efficient epoxidation of allylic alcohols with hydrogen peroxide catalyzed by peroxoniobate-based ionic liquids. ACS Catalysis 2016, 6 (5), 3354-3364.
24. JF Chen; Y Mao; HF Wang; P Hu, Reversibility iteration method for understanding reaction networks and for solving microkinetics in heterogeneous catalysis. ACS Catalysis 2016, 6 (10), 7078-7087.
23. Y Mao; JF Chen; HF Wang; P Hu, Catalyst screening: Refinement of the origin of the volcano curve and its implication in heterogeneous catalysis. Chinese Journal of Catalysis 2015, 36 (9), 1596-1605.
22. Z Wang; HF Wang; P Hu, Possibility of designing catalysts beyond the traditional volcano curve: a theoretical framework for multi-phase surfaces. Chemical science 2015, 6 (10), 5703-5711.
21. Y Mao; HF Wang; P Hu, Theoretical investigation of NH3‐SCR processes over zeolites: A review. International Journal of Quantum Chemistry 2015, 115 (10), 618-630.
20. C Peng; HF Wang; P Hu, Theoretical insights into how the first C-C bond forms in the methanol-to-olefin process catalysed by HSAPO-34. Physical Chemistry Chemical Physics 2016, 18 (21), 14495-14502.
19. YH Zhang; YF Cai; Y Guo; HF Wang; L Wang; Y Lou; YL Guo; GZ Lu; YQ Wang, The effects of the Pd chemical state on the activity of Pd/Al2O3 catalysts in CO oxidation. Catalysis Science & Technology 2014, 4 (11), 3973-3980.
18. XQ Gong; LL Yin; J Zhang; HF Wang; XM Cao; GZ Lu; P Hu, Computational Simulation of Rare Earth Catalysis. Advances in Chemical Engineering 2014, 44, 1-60.
17. YH Li; J Xing; ZJ Chen; Z Li; F Tian; LR Zheng; HF Wang; P Hu; HJ Zhao; HG Yang, Unidirectional suppression of hydrogen oxidation on oxidized platinum clusters. Nature communications 2013, 4, 2500.
16. B Yang; XQ Gong; HF Wang; XM Cao; JJ Rooney; P Hu, Evidence to challenge the universality of the Horiuti-Polanyi mechanism for hydrogenation in heterogeneous catalysis: origin and trend of the preference of a non-Horiuti-Polanyi Mechanism. Journal of the American Chemical Society 2013, 135 (40), 15244-15250.
15. J Xing; HF Wang; C Yang; D Wang; HJ Zhao; GZ Lu; P Hu; HG Yang, Ceria Foam with Atomically Thin Single-Crystal Walls. Angewandte Chemie 2012, 124 (15), 3671-3675.
14. HF Wang; R Kavanagh; YL Guo; Y Guo; GZ Lu; P Hu, Origin of extraordinarily high catalytic activity of Co3O4 and its morphological chemistry for CO oxidation at low temperature. Journal of catalysis 2012, 296, 110-119.
13. HF Wang; R Kavanagh; YL Guo; Y Guo; GZ Lu; P Hu, Structural origin: water deactivates metal oxides to CO oxidation and promotes low‐temperature CO oxidation with metals. Angewandte Chemie International Edition 2012, 51 (27), 6657-6661.
12. HF Wang; HY Li; XQ Gong; YL Guo; GZ Lu; P Hu, Oxygen vacancy formation in CeO2 and Ce1-xZrxO2 solid solutions: electron localization, electrostatic potential and structural relaxation. Physical Chemistry Chemical Physics 2012, 14 (48), 16521-16535.
11. WJ Xu; HF Wang; XH Liu; JW Ren; YQ Wang; GZ Lu, Direct catalytic conversion of furfural to 1,5-pentanediol by hydrogenolysis of the furan ring under mild conditions over Pt/Co2AlO4 catalyst. Chemical Communications 2011, 47 (13), 3924-3926.
10. HY Li; HF Wang; YL Guo; GZ Lu; P Hu, Exchange between sub-surface and surface oxygen vacancies on CeO2(111): a new surface diffusion mechanism. Chemical Communications 2011, 47 (21), 6105-6107.
9. Y Chen; HF Wang; R Burch; C Hardacre; P Hu, New insight into mechanisms in water-gas-shift reaction on Au/CeO2(111): A density functional theory and kinetic study. Faraday discussions 2011, 152, 121-133.
8. HF Wang; YL Guo; GZ Lu; P Hu, Maximizing the Localized Relaxation: The Origin of the Outstanding Oxygen Storage Capacity of κ‐Ce2Zr2O8. Angewandte Chemie International Edition 2009, 48 (44), 8289-8292
7. HF Wang; YL Guo; G Lu; P Hu, NO oxidation on platinum group metals oxides: first principles calculations combined with microkinetic analysis. The Journal of Physical Chemistry C 2009, 113 (43), 18746-18752.
6. HF Wang; YL Guo; GZ Lu; P Hu, An understanding and implications of the coverage of surface free sites in heterogeneous catalysis. The Journal of chemical physics 2009, 130 (22), 224701.
5. HF Wang; XQ Gong; YL Guo; Y Guo; G Lu; P Hu, Structure and catalytic activity of gold in low-temperature CO oxidation. The Journal of Physical Chemistry C 2009, 113 (15), 6124-6131.
4. HF Wang; XQ Gong; YL Guo; Y Guo; GZ Lu; P Hu, A Model to Understand the Oxygen Vacancy Formation in Zr-Doped CeO2: Electrostatic Interaction and Structural Relaxation. The Journal of Physical Chemistry C 2009, 113 (23), 10229-10232.
3. HY Li; HF Wang; XQ Gong; YL Guo; Y Guo; GZ Lu; P Hu, Multiple configurations of the two excess 4f electrons on defective CeO2(111): Origin and implications. Physical Review B 2009, 79 (19), 193401.
2. Y Chen; P Hu; MH Lee; HF Wang, Au on (111) and (110) surfaces of CeO2: A density-functional theory study. Surface Science 2008, 602 (10), 1736-1741
1. Y Chen; J Cheng; P Hu; HF Wang, Examining the redox and formate mechanisms for water-gas shift reaction on Au/CeO2 using density functional theory. Surface science 2008, 602 (17), 2828-2834.
Photocatalysis and Electrocatalysis
The rapid increase in global energy demand and serious environmental disasters have driven interest in developing renewable energy sources to replace traditional fossil fuels, which further promotes the innovation on green chemistry. Therefore, our group have been engaged in first principle calculations on photocatalysis, electrocatalysis and solar cells, with the following being the brief introduction.
In terms of photocatalysis, we investigated the microscopic mechanism of HER and OER in detail. We scrutinized the unique photocatalytic properties of rutile TiO2(110) and the role of photogenerated holes in photocatalytic surface reactions. In addition, CH3NH3PbI3 perovskite and its light-induced degradation were discussed.
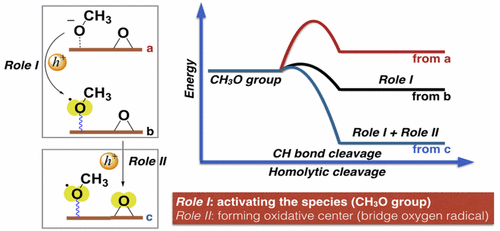
When it comes to electrocatalysis, the surface-confined electrochemical behavior of single nanoparticles at interfaces was explored. We turned the local atomic structure to boost HER in acidic water. Such strategy was used to modify the metal-C3N4 interaction in CO2RR. Additionally, the importance of activated adsorbates was emphasized by DFT calculations in neutral OER.
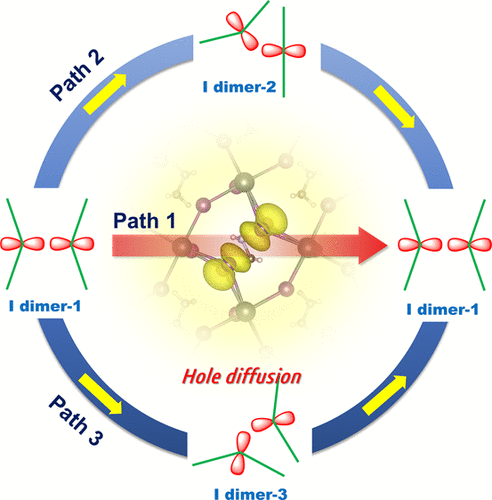
Finally, dye-sensitized solar cells are promising choice for green energy conversion process. We have screened out the low-cost counter electrodes and investigated properties of cosensitized porphyrin system. With regard to the important I3– reduction reaction, we revealed its volcano curve and its facet-dependent catalytic activity of platinum nanocrystals. RuO2, heteroatom dopped graphene and InO2 possesses the outstanding catalytic reactivity in this reaction.
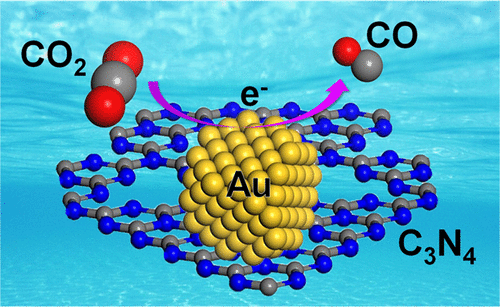
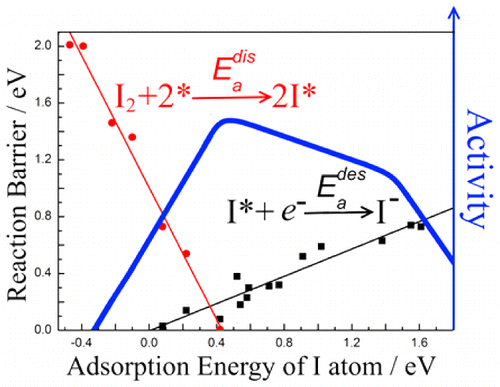
Publications
42. XL Xu; XJ Song; XH Liu; HF Wang; YF Hu; J Xia; JC Chen; M Shakouri; Y Guo; YQ Wang, A Highly Efficient Nickel Phosphate Electrocatalyst for the Oxidation of 5-Hydroxymethylfurfural to 2,5-Furandicarboxylic Acid. ACS Sustainable Chem. Eng. 2022, 10 (17), 5538-5547.
41. XY Zhang; WJ Li; JC Chen; XF Wu; YW Liu; FX Mao; HY Yuan; MH Zhu; S Dai; HF Wang; P Hu; CH Sun; PF Liu; HG Yang, Operando Metalloid Znδ+ Active Sites for Highly Efficient Carbon Dioxide Reduction Electrocatalysis. Angew. Chem. Int. Ed. 2022, e202202298.
40. HQ Fu; M Zhou; PF Liu; PR Liu; HJ Yin; KZ Sun; HG Yang; M Al-Mamun; PJ Hu; HF Wang; HJ Zhao, Hydrogen Spillover-Bridged Volmer/Tafel Processes Enabling Ampere-Level Current Density Alkaline Hydrogen Evolution Reaction under Low Overpotential. J. Am. Chem. Soc. 2022, 144 (13), 6028-6039.
39. M Zhou; HF Wang, Insight into the photoexcitation effect on the catalytic activation of H2 and CH bonds on TiO2(110) surface. Chinese Chemical Letters 2022.
38. ZX Lou; WJ Li; HY Yuan; Y Hou; HG Yang; HF Wang, Structural rule of N-coordinated single-atom catalysts for electrochemical CO2 reduction. J. Mater. Chem. A, 2022, 10, 3585.
37. XY Zhang; WJ Li; XF Wu; YW Liu; JC Chen; MH Zhu; HY Yuan; S Dai; HF Wang; Z Jiang; PF Liu; HG Yang, Selective methane electrosynthesis enabled by a hydrophobic carbon coated copper core-shell architecture. Energy Environ. Sci. 2022, 15, 234-243.
36. M Zhou; HF Wang, Optimally Selecting Photo- and Electrocatalysis to Facilitate CH4 Activation on TiO2(110) Surface: Localized Photoexcitation versus Global Electric-Field Polarization. JACS Au 2022, 2 (1), 188-196.
35. CF Wen; M Zhou; PF Liu; YW Liu; XF Wu; FX Mao; S Dai; BB Xu; XL Wang; Z Jiang; P Hu; S Yang; HF Wang; HG Yang, Highly Ethylene-Selective Electrocatalytic CO2 Reduction Enabled by Isolated Cu-S Motifs in Metal-Organic Framework Based Precatalysts. Angew. Chem. Int. Ed. 2022, 61, e202111700.
34. YW Liu; LJ Wang; H Zhang; HY Yuan; QH Zhang; L Gu; HF Wang; P Hu; PF Liu; Z Jiang; HG Yang, Boosting Photocatalytic Water Oxidation Over Bifunctional Rh0-Rh3+ Sites. Angew. Chem. Int. Ed. 2021,60, 22761-22768.
33. SM Lu; JF Chen; YY Peng; W Ma; H Ma; HF Wang; PJ Hu; YT Long, Understanding the Dynamic Potential Distribution at the Electrode Interface by Stochastic Collision Electrochemistry. J. Am. Chem. Soc. 2021, 143 (32), 12428-12432.
32. LS Zhang; HY Yuan; LP Wang; H Zhang; YJ Zang; Y Tian, YZ Wen; FL Ni; H Song; HF Wang; B Zhang; HS Peng, The critical role of electrochemically activated adsorbates in neutral OER. Sci China Mater 2020, 63(12), 2509-2516.
31. WH Chen, JH Xiong, X Teng, JX Mi, ZB Hu, HF Wang, ZF Chen, A novel heterogeneous Co(II)-Fenton-like catalyst for efficient photodegradation by visible light over extended pH. Sci China Chem 2020, 63 (12), 1825-1836.
30. XJ Song; XH Liu; HF Wang; Y Guo; YQ Wang, Improved Performance of Nickel Boride by Phosphorus Doping as an Efficient Electrocatalyst for the Oxidation of 5-Hydroxymethylfurfural to 2,5-Furandicarboxylic Acid. Ind. Eng. Chem. Res. 2020, 59 (39), 17348-17356.
29. WC Qiao; JW Wu; R Zhang; WO Yang; XH Chen; G Yang; Q Chen; XL Wang; HF Wang; YF Yao, In situ NMR Investigation of the Photoresponse of Perovskite Crystal. Matter (in press) http://dx.doi.org/10.2139/ssrn.3575166
28. H Ma; JF Chen; HF Wang; P Hu; W Ma; YT Long, Exploring dynamic interactions of single nanoparticles at interfaces for surface-confined electrochemical behavior and size measurement. Nature communications 2020, 11 (1), 1-9.
27. LS Zhang; HY Yuan; LP Wang; H Zhang; YJ Zang; Y Tian; YZ Wen; FL Ni; H Song; HF Wang; B Zhang; HS Peng, The critical role of electrochemically activated adsorbates in neutral OER. Science China Materials 2020, 1-8.
26. BB Xu; M Zhou; R Zhang; M Ye; LY Yang; R Huang; HF Wang; XL Wang; YF Yao, Solvent Water Controls Photocatalytic Methanol Reforming. The Journal of Physical Chemistry Letters 2020, 11 (9), 3738-3744.
25. D Wang; T Sheng; JF Chen; HF Wang; P Hu, Identifying the key obstacle in photocatalytic oxygen evolution on rutile TiO2. Nature Catalysis 2018, 1 (4), 291-299.
24. L Zhang; F Mao; LR Zheng; HF Wang; XH Yang; HG Yang, Tuning Metal Catalyst with Metal-C3N4 Interaction for Efficient CO2 Electroreduction. ACS Catalysis 2018, 8 (12), 11035-11041.
23. HL Song; J Zhang; JM Jin; HF Wang; YS Xie, Porphyrin sensitizers with modified indoline donors for dye-sensitized solar cells. Journal of Materials Chemistry C 2018, 6 (15), 3927-3936.
22. C Peng; G Reid; HF Wang; P Hu, Perspective: Photocatalytic reduction of CO2 to solar fuels over semiconductors. The Journal of Chemical Physics 2017, 147 (3), 030901.
21. C Peng; JL Wang; HF Wang; P Hu, Unique Trapped Dimer State of the Photogenerated Hole in Hybrid Orthorhombic CH3NH3PbI3 Perovskite: Identification, Origin, and Implications. Nano letters 2017, 17 (12), 7724-7730.
20. WJ Wu; HD Xiang; W Fan; JL Wang; HF Wang; X Hua; ZH Wang; YT Long; H Tian; WH Zhu, Cosensitized porphyrin system for high-performance solar cells with TOF-SIMS analysis. ACS Applied Materials & Interfaces 2017, 9 (19), 16081-16090.
19. C Peng; JF Chen; HF Wang; P Hu, First-Principles Insight into the Degradation Mechanism of CH3NH3PbI3 Perovskite: Light-Induced Defect Formation and Water Dissociation. The Journal of Physical Chemistry C 2018, 122 (48), 27340-27349.
18. JW Zhang; C Peng; HF Wang; P Hu, Identifying the Role of Photogenerated Holes in Photocatalytic Methanol Dissociation on Rutile TiO2(110). ACS Catalysis 2017, 7 (4), 2374-2380.
17. JF Chen; Y Mao; HF Wang; P Hu, Theoretical study of heteroatom doping in tuning the catalytic activity of graphene for triiodide reduction. ACS Catalysis 2016, 6 (10), 6804-6813.
16. L Qian; JF Chen; YH Li; L Wu; HF Wang; AP Chen; P Hu; LR Zheng; HG Yang, Orange Zinc Germanate with Metallic Ge-Ge Bonds as a Chromophore‐Like Center for Visible-Light-Driven Water Splitting. Angewandte Chemie 2015, 127 (39), 11629-11633.
15. L Wang; M Al-Mamun; P Liu; Y Wang; HG Yang; HF Wang; H Zhao, The search for efficient electrocatalysts as counter electrode materials for dye-sensitized solar cells: mechanistic study, material screening and experimental validation. NPG Asia Materials 2015, 7 (11), e226.
14. YH Li; PF Liu; LF Pan; HF Wang; ZZ Yang; LR Zheng; P Hu; HJ Zhao; L Cu; HG Yang, Local atomic structure modulations activate metal oxide as electrocatalyst for hydrogen evolution in acidic water. Nature communications 2015, 6, 8064.
13. H Jiang; DY Ren; HF Wang; YJ Hu; SJ Guo; HY Yuan; P Hu; L Zhang; CZ Li, 2D Monolayer MoS2-Carbon Interoverlapped Superstructure: Engineering Ideal Atomic Interface for Lithium Ion Storage. Advanced Materials 2015, 27 (24), 3687-3695.
12. YH Li; C Peng; S Yang; HF Wang; HG Yang, Critical roles of co-catalysts for molecular hydrogen formation in photocatalysis. Journal of Catalysis 2015, 330, 120-128.
11. D Wang; HF Wang; P Hu, Identifying the distinct features of geometric structures for hole trapping to generate radicals on rutile TiO2(110) in photooxidation using density functional theory calculations with hybrid functional. Physical Chemistry Chemical Physics 2015, 17 (3), 1549-1555.
10. J Xing; JF Chen; YH Li; WT Yuan; Y Zhou; LR Zheng; HF Wang; P Hu; Y Wang; HJ Zhao; Y Wang; HG Yang, Stable isolated metal atoms as active sites for photocatalytic hydrogen evolution. Chemistry-A European Journal 2014, 20 (8), 2138-2144.
9. Y Hou; ZP Chen; D Wang; B Zhang; S Yang; HF Wang; P Hu; HJ Zhao; HG Yang, Highly Electrocatalytic Activity of RuO2 Nanocrystals for Triiodide Reduction in Dye-Sensitized Solar Cells. Small 2014, 10 (3), 484-492.
8. YB Li; HF Wang; HM Zhang; PR Liu; Y Wang; WQ Fang; HG Yang; Y Li; HJ Zhao, A {0001} faceted single crystal NiS nanosheet electrocatalyst for dye-sensitised solar cells: sulfur-vacancy induced electrocatalytic activity. Chemical communications 2014, 50 (42), 5569-5571.
7. J Xing; HB Jiang; JF Chen; YH Li; L Wu; S Yang; LR Zheng; HF Wang; P Hu; HJ Zhao; HG Yang, Active sites on hydrogen evolution photocatalyst. Journal of Materials Chemistry A 2013, 1 (48), 15258-15264.
6. Yu Hang Li, Jun Xing, Zong Jia Chen, Zhen Li, Feng Tian, Li Rong Zheng, Hai Feng Wang, P Hu, Hui Jun Zhao, Hua Gui Yang, Unidirectional suppression of hydrogen oxidation on oxidized platinum clusters. Nature communications 2013, 4, 2500
5. Y Hou; D Wang; XH Yang; WQ Fang; B Zhang; HF Wang; GZ Lu; P Hu; HJ Zhao; HG Yang, Rational screening low-cost counter electrodes for dye-sensitized solar cells. Nature communications 2013, 4 (1), 1-8.
4. CJ Li; P Zhang; R Lv; JW Lu; T Wang; SP Wang; HF Wang; JL Gong, Selective deposition of Ag3PO4 on monoclinic BiVO4(040) for highly efficient photocatalysis. Small 2013, 9 (23), 3951-3956.
3. B Zhang; NN Zhang; JF Chen; Y Hou; S Yang; JW Guo; XH Yang; JH Zhong; HF Wang; P Hu; HJ Zhao; HG Yang, Turning indium oxide into a superior electrocatalyst: deterministic heteroatoms. Scientific reports 2013, 3, 3109.
2. B Zhang; D Wang; Y Hou; S Yang; XH Yang; JH Zhong; J Liu; HF Wang; P Hu; HJ Zhao; HG Yang, Facet-dependent catalytic activity of platinum nanocrystals for triiodide reduction in dye-sensitized solar cells. Scientific reports 2013, 3, 1836.
1. XL Wang; WQ Fang; HF Wang; H Zhang; H Zhao; Y Yao; HG Yang, Surface hydrogen bonding can enhance photocatalytic H2 evolution efficiency. Journal of materials chemistry A 2013, 1 (45), 14089-14096.
Machine Learning
Hampered by the huge configuration space, the traditional computational chemistry method, especially ab-initio molecular dynamics (AIMD) could not bridge time gap and space gap, which dramatically limits its application in rational catalyst design. In the past decade, artificial intelligence became increasingly popular and machine learning rode on the crest of this wave. Nascent machine-learning techniques could accurately fit the high-dimensional potential field and the iterative calculation could be exponentially accelerated, whereby the bottleneck of basic DFT calculation processes could be broken through. Recently, we applied deep learning techniques in catalysis. The details are listed as follows:
We developed an on-the-fly training strategy combing the an advanced neural network called SchNet and the genetic algorithm (GA) search technique to rapidly figure out the correlation between configuration and energy of 38-atom Pt-Au cluster, whose accuracy could reach DFT level. Interestingly, the thermodynamically most stable PtmAu38-m cluster is Pt6Au32, with all the core-region sites occupied by Pt.

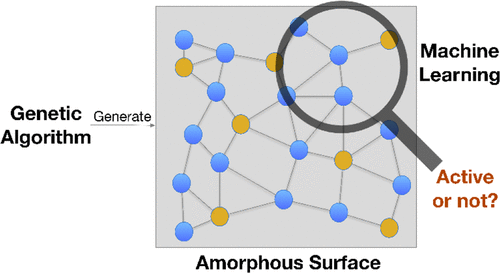
By virtue of GBDT algorithm and HDNN, we successfully screened out the highly active site of amorphous Ni2P catalyst for HER. Our method decomposes the difficult prediction of adsorption energy on amorphous surfaces into two sub-problems: frozen adsorption energy and relaxation energy. By training with a set of ab-initio adsorption energies within a wide configuration space, we succeed to predict the adsorption energies with ~0.1 eV error by adopting the feature only relying on local chemical environment.
Publications
4. DM Chen; ZZ Lai; JW Zhang; JF Chen; PJ Hu; HF Wang, Gold Segregation Improves Electrocatalytic Activity of Icosahedron Au@Pt Nanocluster: Insights from Machine Learning. Chin. J. Chem. 2021, 39, 3029-3036.
3. J Zhang; J Chen; P Hu; H Wang, Identifying the composition and atomic distribution of Pt-Au bimetallic nanoparticle with machine learning and genetic algorithm. Chinese Chemical Letters 2020, 31 (3), 890-896.
2. J Zhang; P Hu; H Wang, Amorphous Catalysis: Machine Learning Driven High-Throughput Screening of Superior Active Site for Hydrogen Evolution Reaction. The Journal of Physical Chemistry C 2020, 124 (19), 10483-10494.
1. C Zhou; B Zhang; P Hu; HF Wang, An effective structural descriptor to quantify the reactivity of lattice oxygen in CeO2 Subnano-clusters. Physical Chemistry Chemical Physics 2020, 22 (3), 1721-1726.
Background
As a core technology, catalysis has made great contributions to many aspects such as ammonia synthesis, environmental protection, chemical production, petroleum processing, efficient utilization of energy and resources, etc. The purpose of the present and future research on catalysis is to understand the nature of catalysis at the atomic and molecular level in order to design more economical and efficient catalysts.
Thanks to the development of computer technology, computational catalysis based on Density Functional Theory (DFT) can establish a bridge between catalytic theory and catalytic practice, and solve some practical problems in catalytic research. In addition, it can also reduce the repetitive labor in catalytic research, thereby reducing the consumption of manpower and financial resources.
Firstly, DFT calculations have become a powerful tool to investigate catalytic processes. The binding energies of intermediates, reaction barriers, surface structures, and so on can be routinely obtained with reasonable accuracy. However, some challenges still remain, such as the ab initio simulation of liquid–solid interfaces and proper consideration of the inherent errors in DFT. Secondly, with the necessary energies obtained by DFT calculations, kinetic analysis has been increasingly used to assess the performance of catalysts and determine the key rate-limiting factor. In short, the kinetics serves as a bridge between the macroscopic behavior of catalysts and the microscopic reaction pathways.
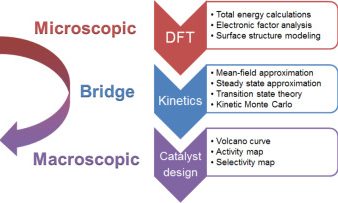
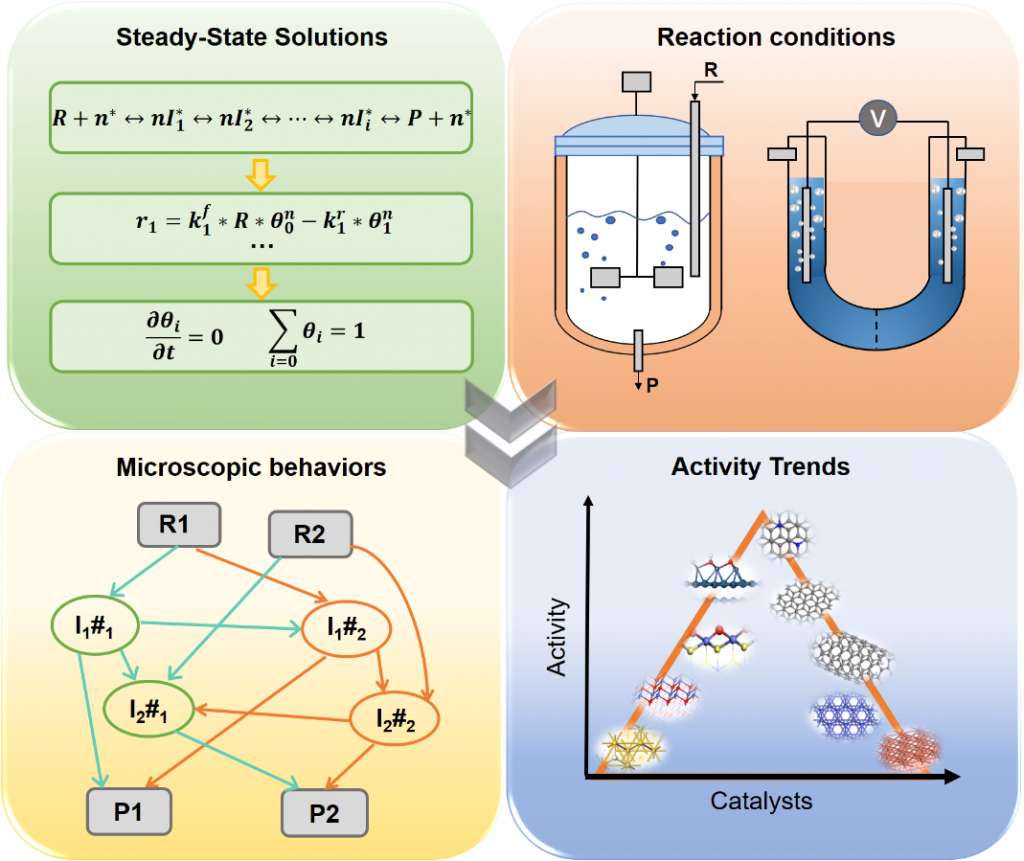
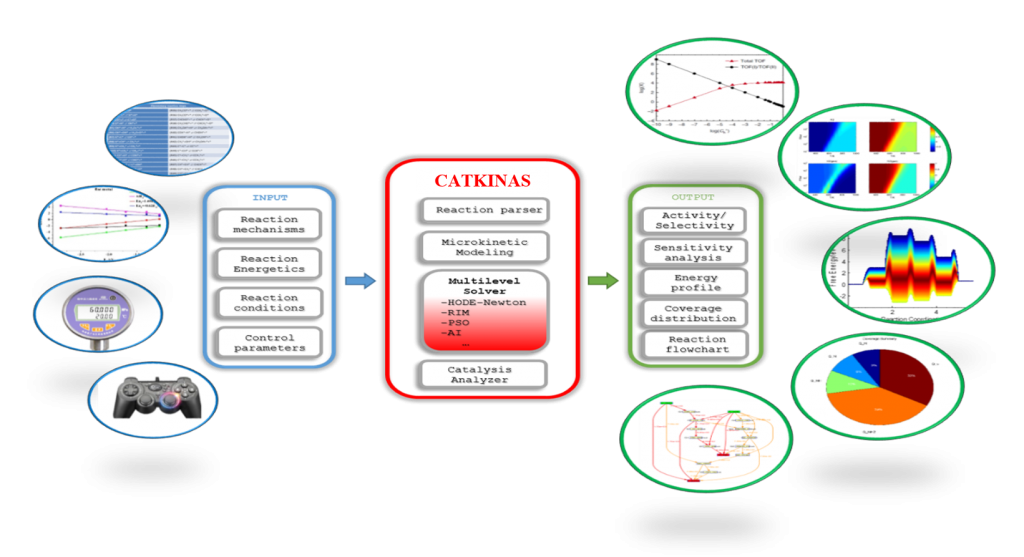
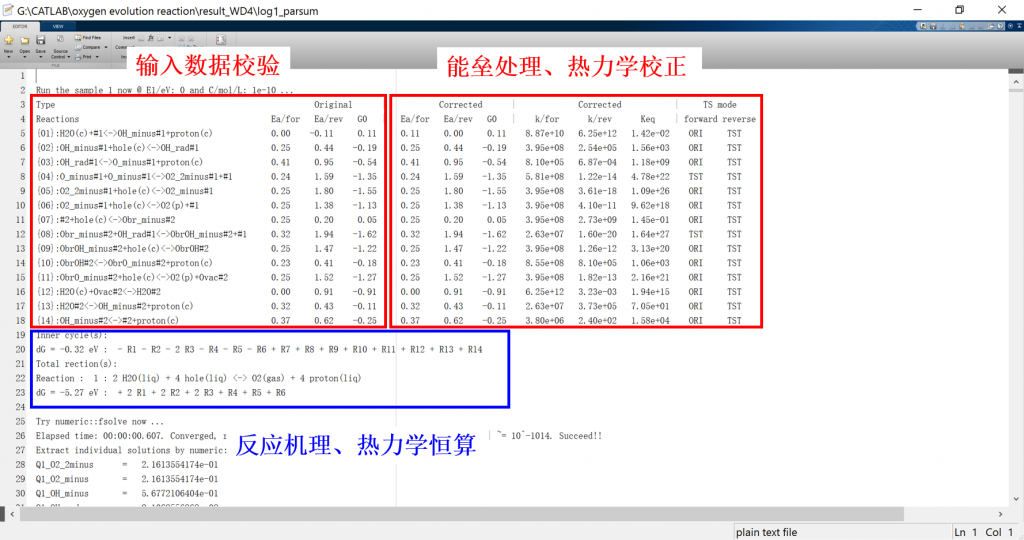
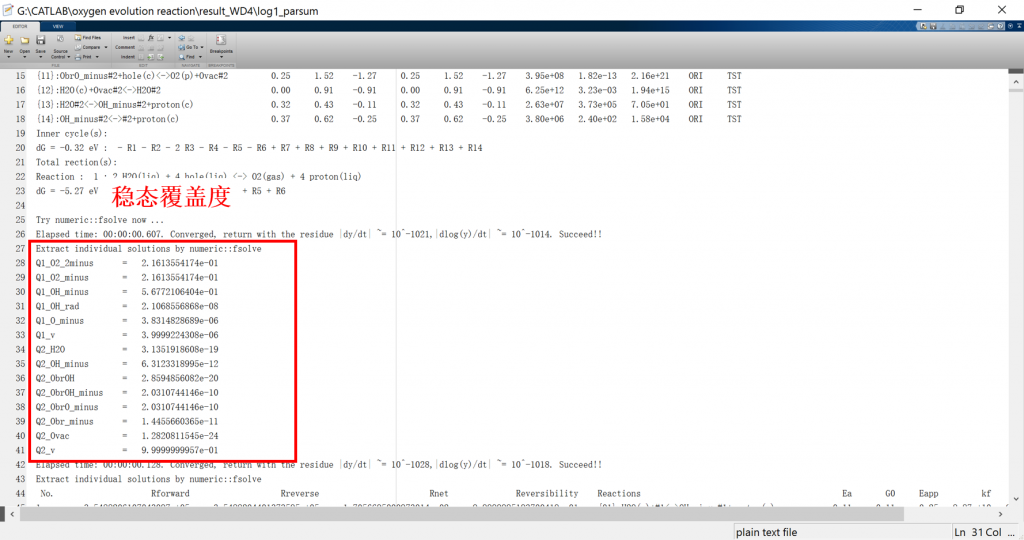
Microkinetic modeling is an effective method to analyze complex catalytic reaction networks and is gaining increasing popularity in the catalytic activity evaluation and rational design of heterogeneous catalysts. As a bridge connecting the microscopic behaviors with macroscopic properties, the microkinetic analysis can solve the steady-state coverages and reaction rates for a wide range of heterogeneous catalytic systems, providing additionally a thorough understanding of the species involved and the activity trends among different catalysts. In this context, the CATKINAS package, as a versatile and user-friendly kinetic tool, is developed by our group. CATKINAS package now includes more than hundreds of subroutines and ten thousand lines of code used for efficiently solving, analyzing, and visualizing the complicated microkinetic properties of various catalytic systems.
Example
Ammonia synthesis
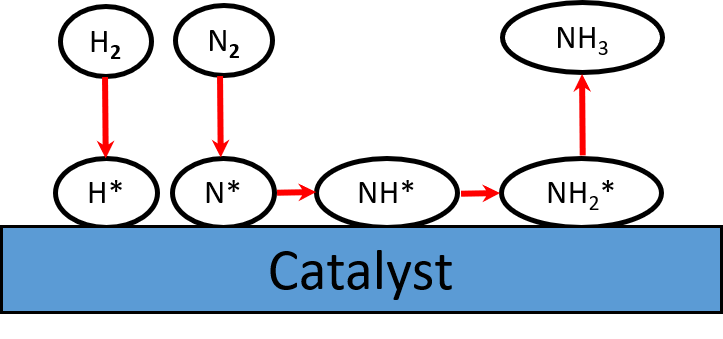
Requirements
•1. Reaction mechanism
•2. Reaction energy
•3. Reaction condition
•4. Control parameter (Optional)
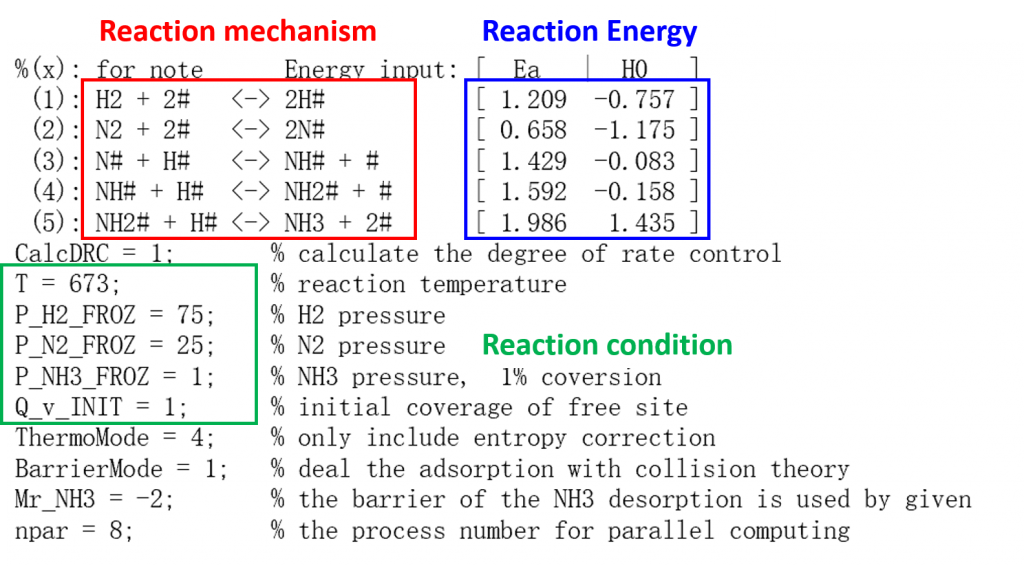
光解水析氧反应
Input files
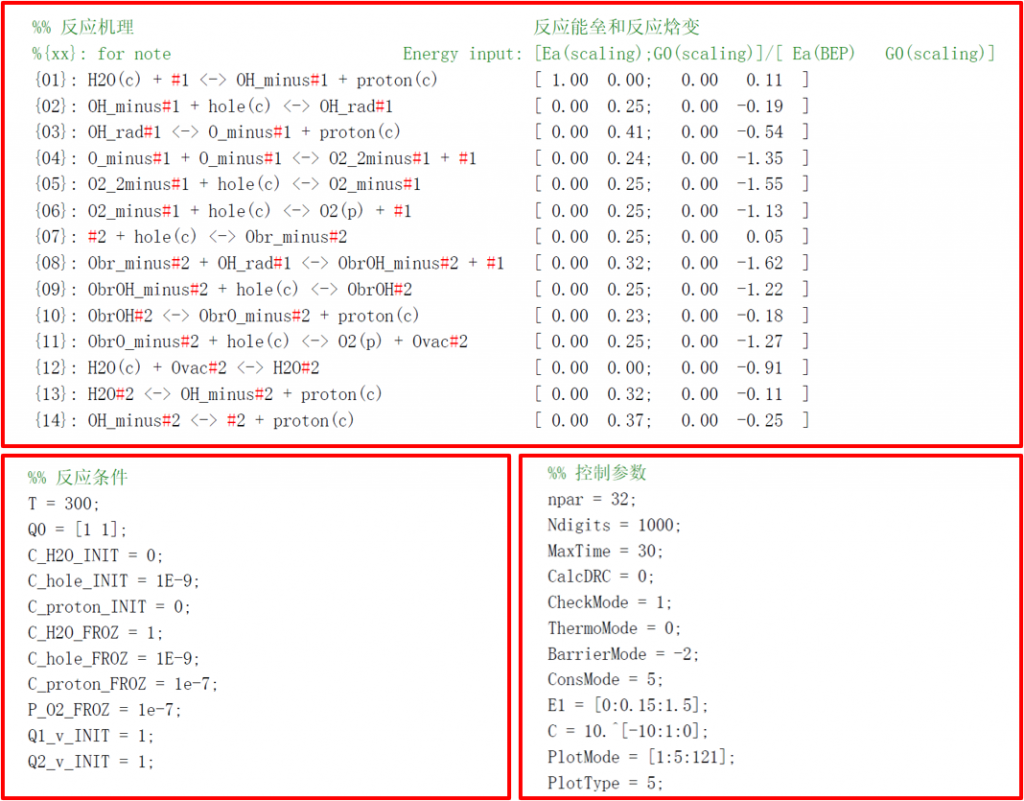
Output files

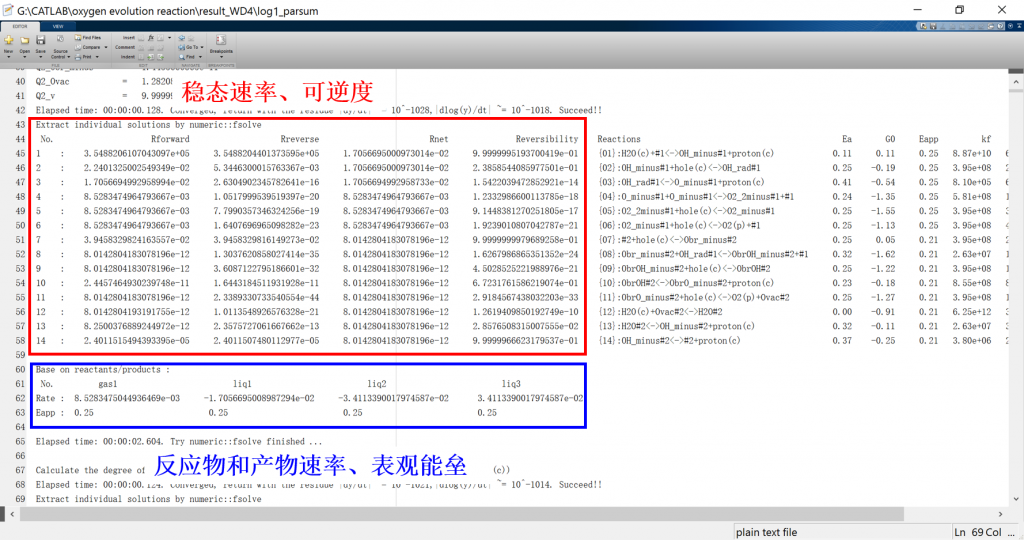
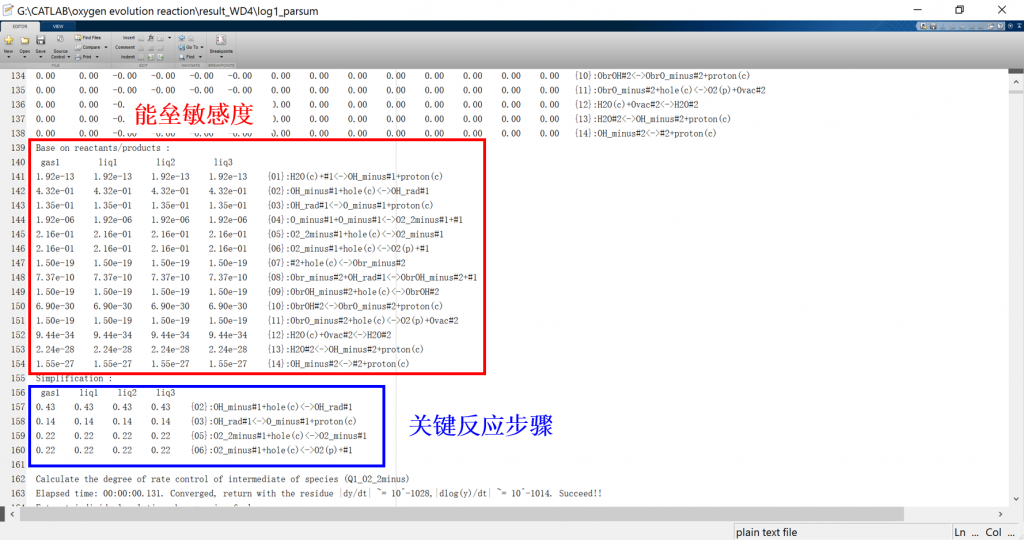
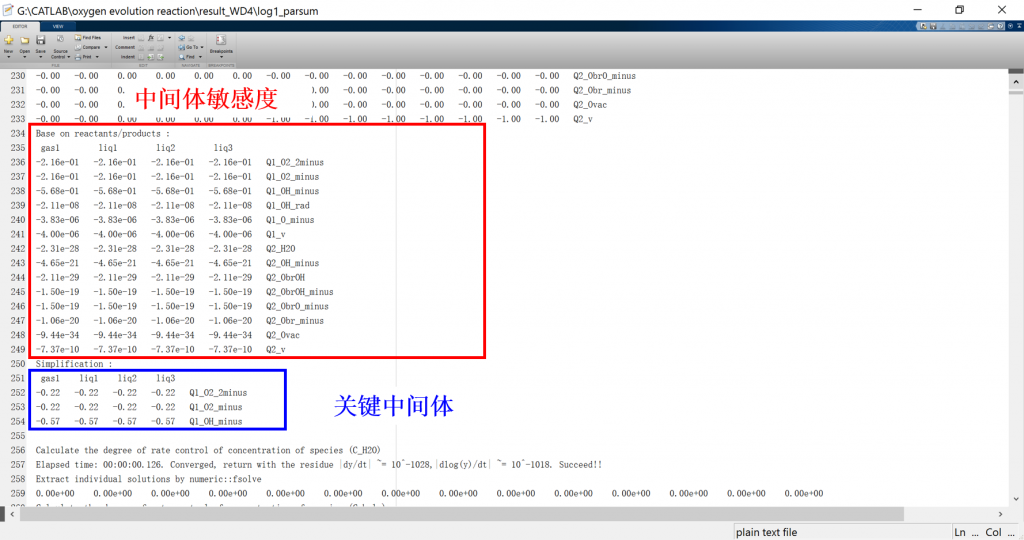
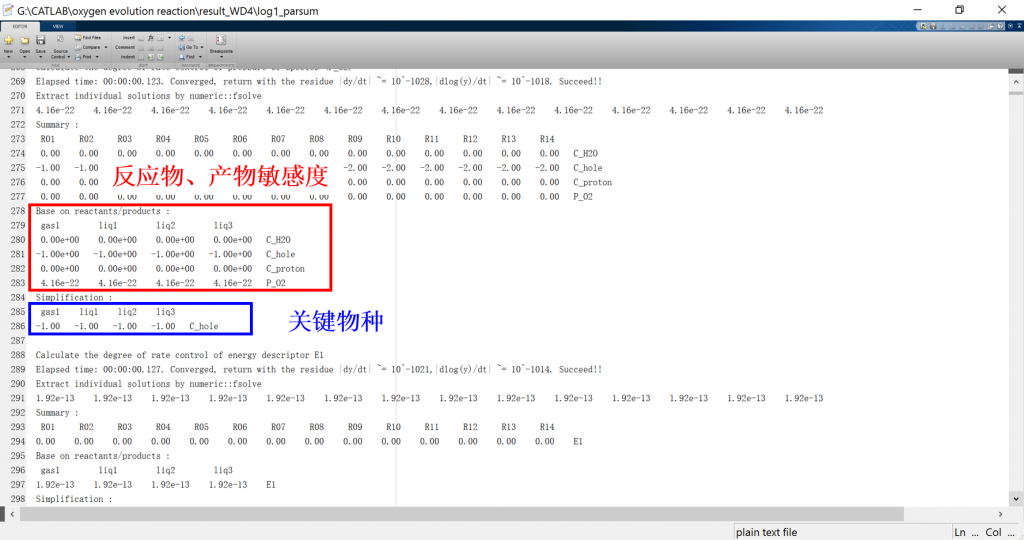

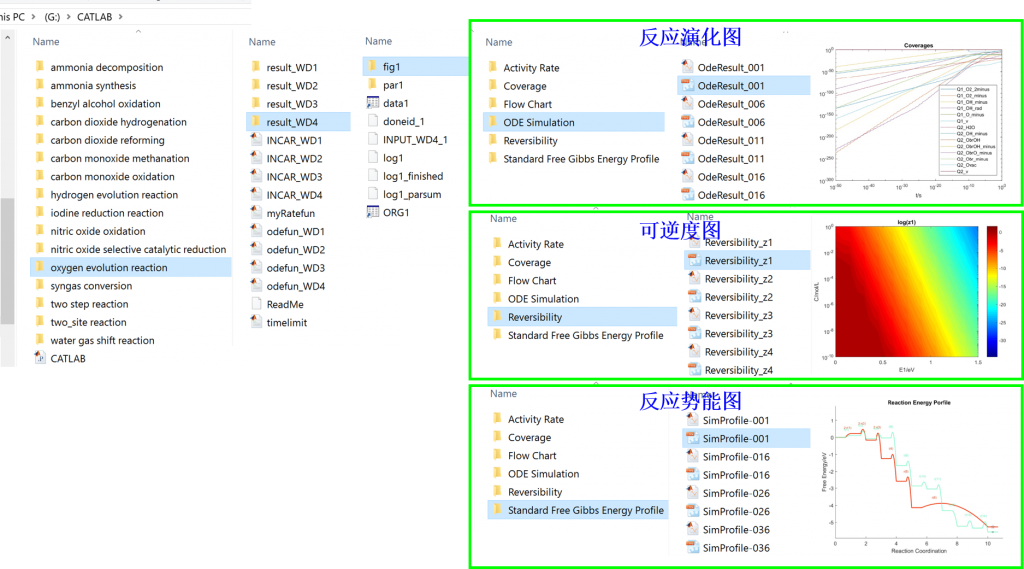
Input/Output files
• CATKINAS basic input files:
1. CATKINAS.in: parameters for simulation, solution and visualization
2. Thermodynamics.data (optional): Thermodynamics data for energy correction
• CATKINAS basic output files:
1. log: run result log in text format (log_parsum for parallel)
2. data.mat: run result data in matrix format
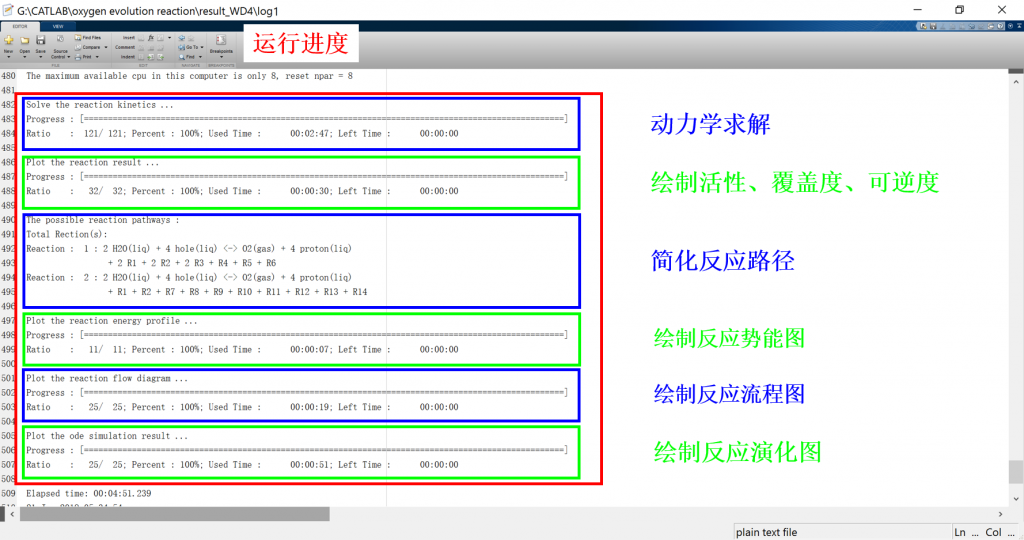
3. fig: visualization result for activity, coverage, reversibility, degree of rate control, energy profile, flow chart, etc.
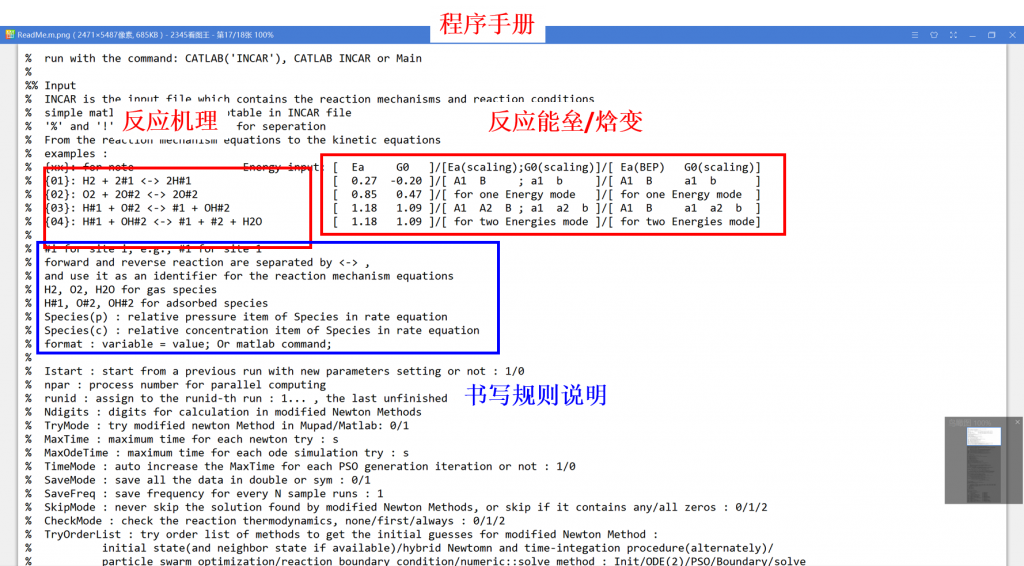
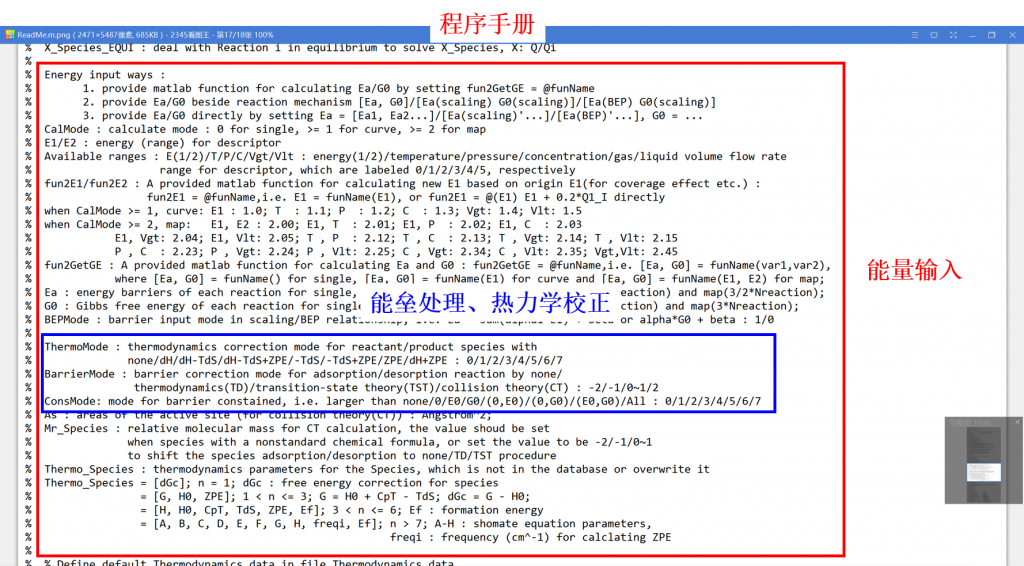
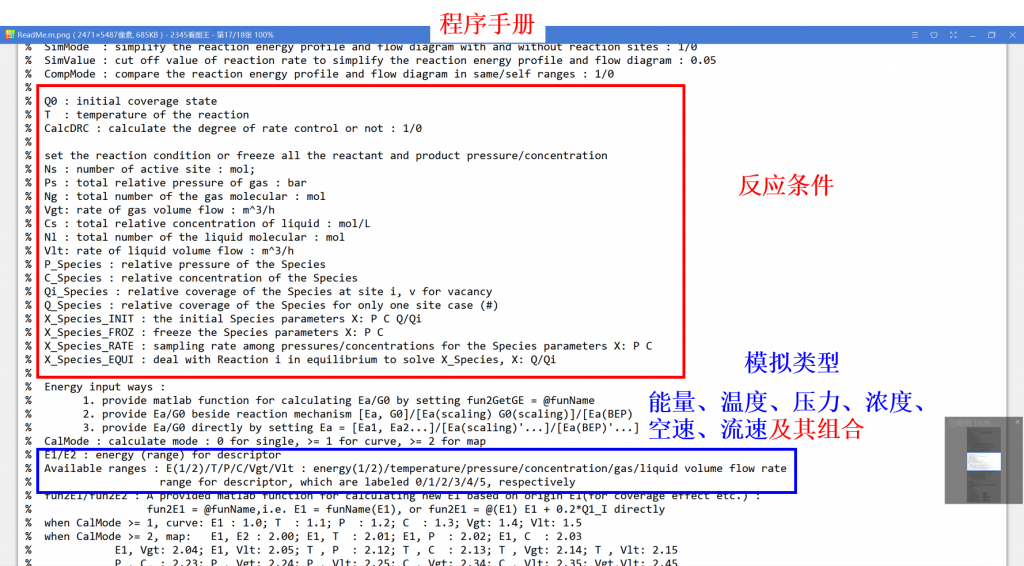
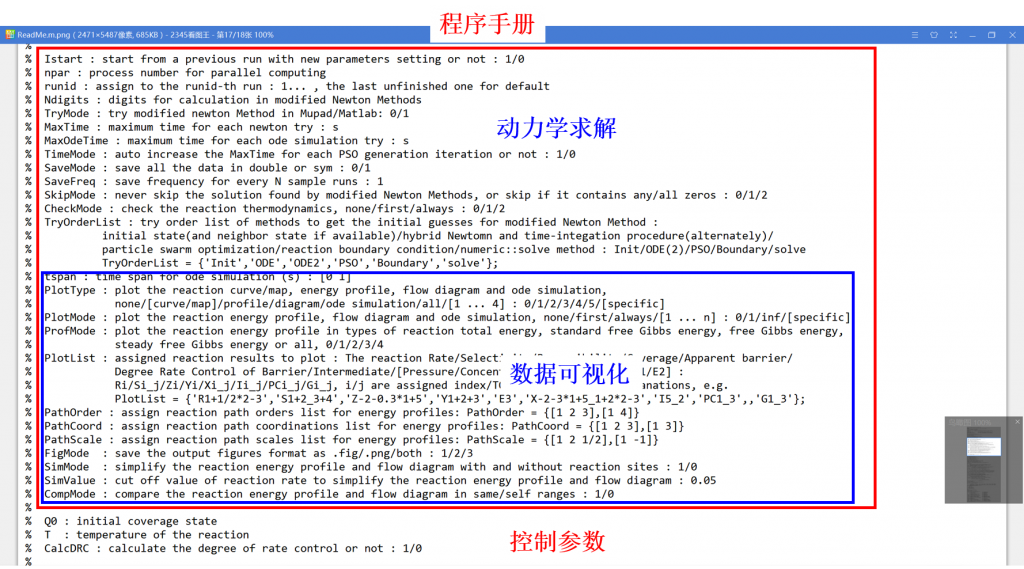
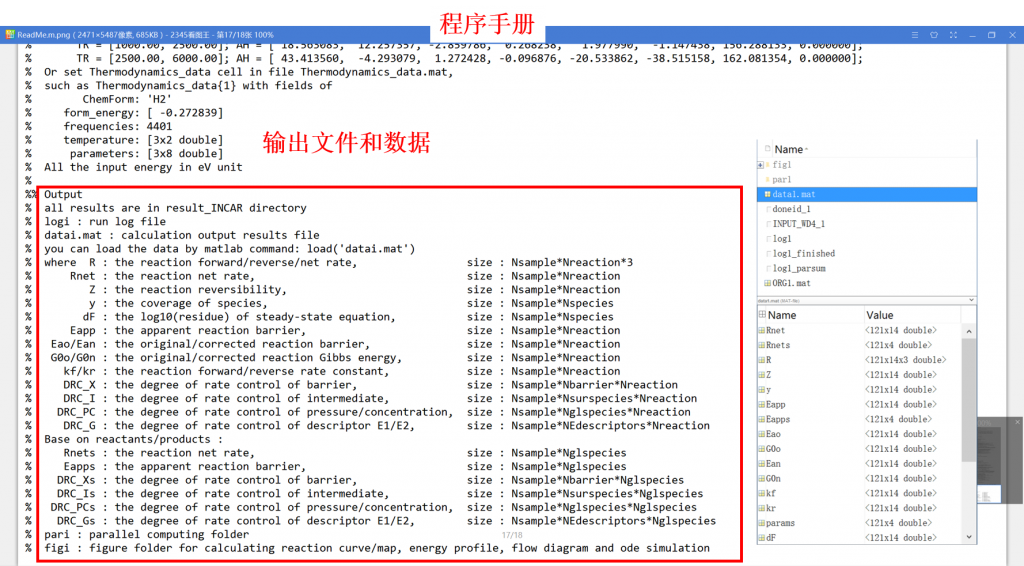
Getting Started
• How to install?
Run in MATLAB (version ≥R2014a)
No additional installation is required!
• How to run?
1. Start MATLAB, and at the prompt type: CATKINAS INPUT
2. Run in the background, save command “CATKINAS INPUT” to the file: Main.m
and run the following command in the shell and cmd window, respectively:
nohup MATLABPATH/matlab <Main.m> print-out &
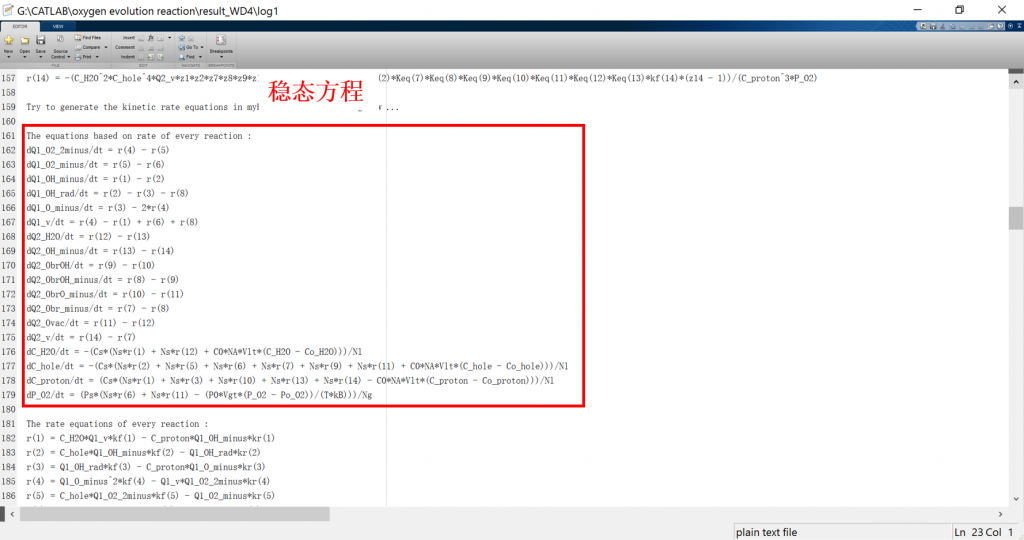
“MATLABPATH\matlab.exe” -nodesktop -nodisplay -nosplash -r “run(‘Main’)”
7 附录
7.1 内置元素周期表参数
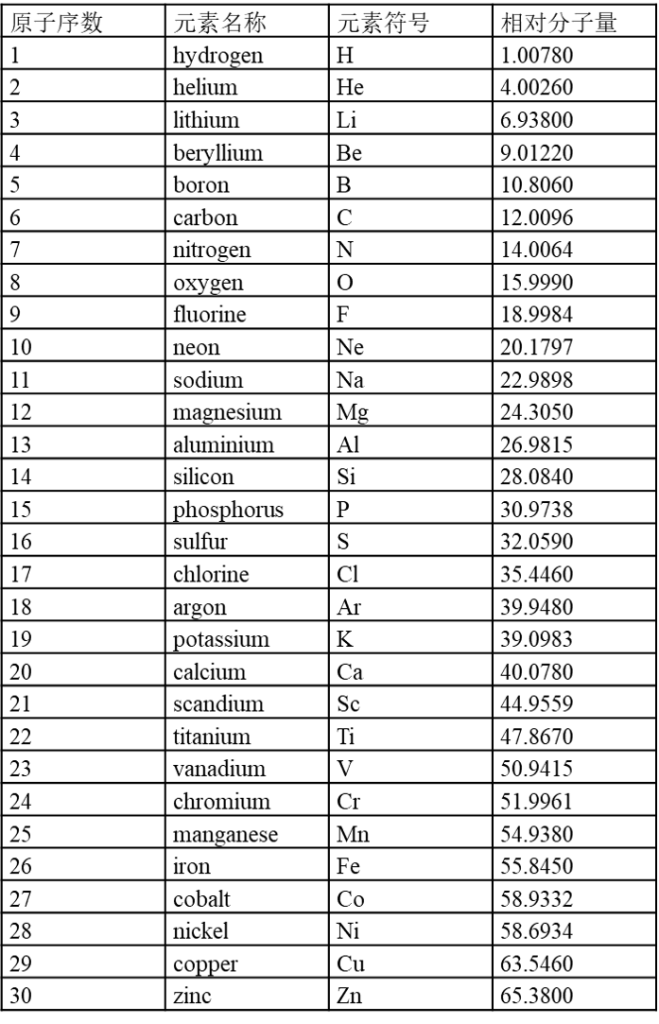
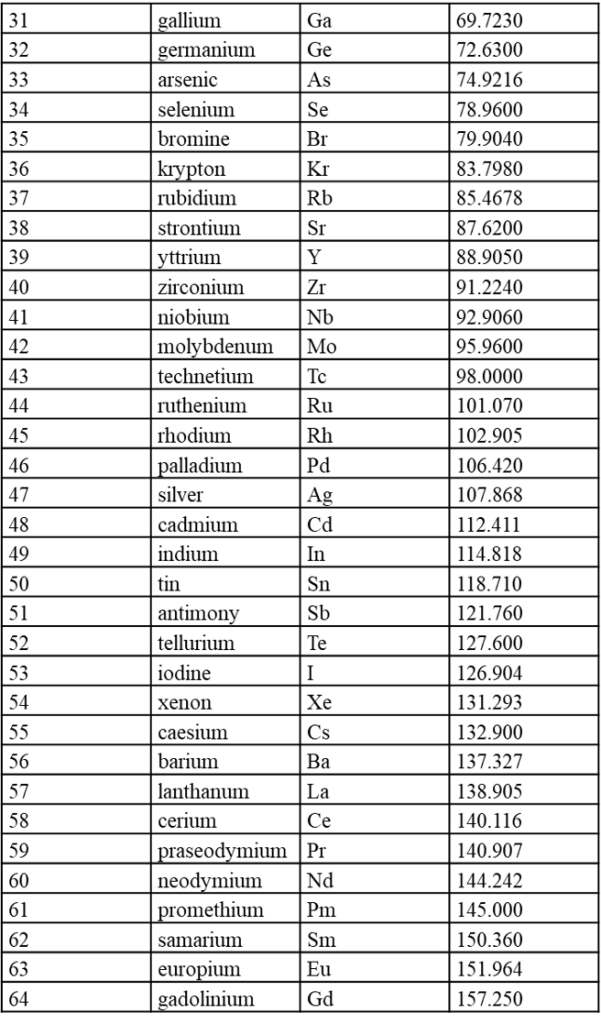
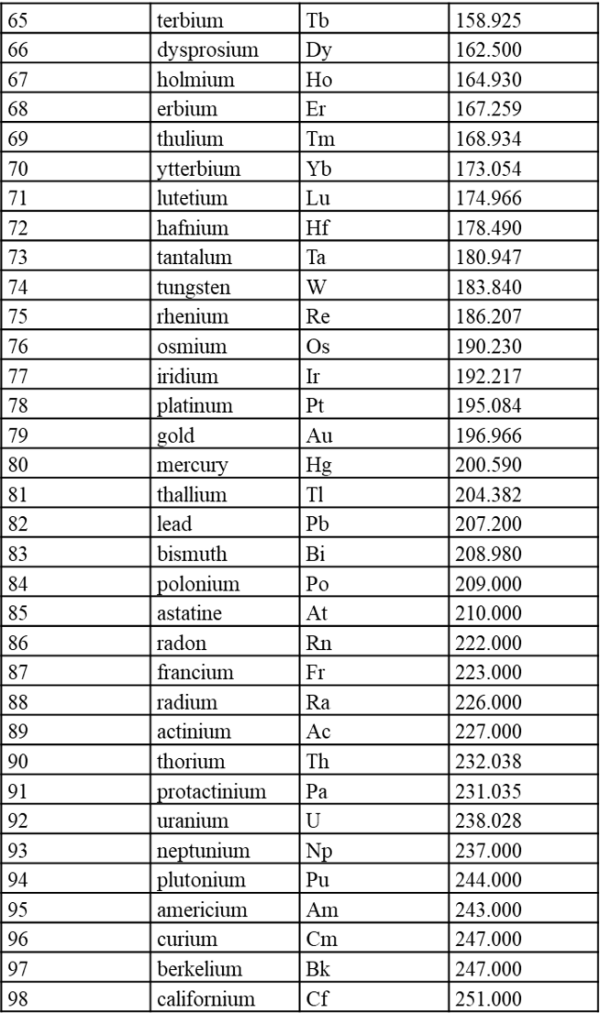
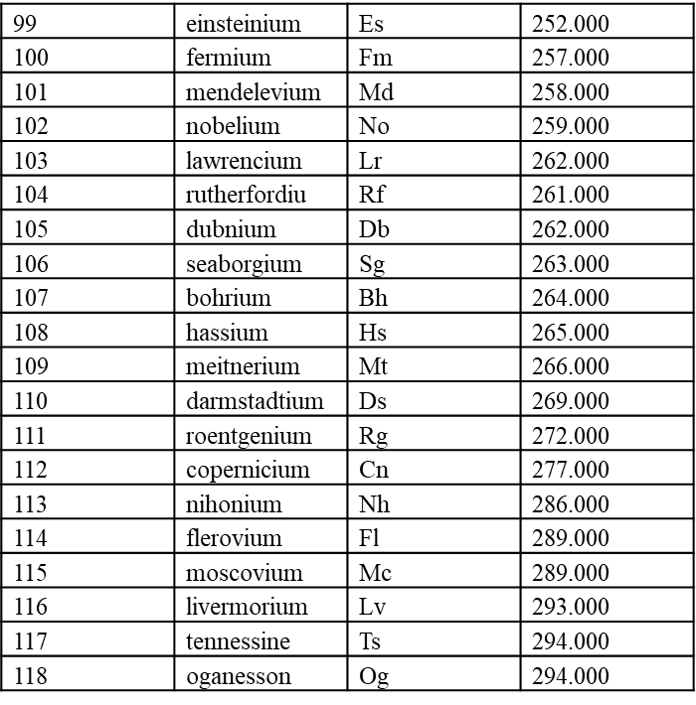
7.2 内置常见分子热力学参数
% Define the default thermodynamics data in the file Thermodynamics.data
% ‘%’ and ‘!’ for comments
% Key words: CF, TR, AH, FQ, EF
% CF: the chemical formula, TR: the temperature range, AH: the shomate parameters [A – H]
% FQ: the frequency, EF: the experimental formation energy
CF = H2; % 1;
EF = [ -0.272839]; FQ = [ 4401.00];
TR = [ 298.00, 1000.00]; AH = [ 33.066178, -11.363417, 11.432816, -2.772874, -0.158558, -9.980797, 172.707974, 0.000000];
TR = [ 1000.00, 2500.00]; AH = [ 18.563083, 12.257357, -2.859786, 0.268238, 1.977990, -1.147438, 156.288133, 0.000000];
TR = [ 2500.00, 6000.00]; AH = [ 43.413560, -4.293079, 1.272428, -0.096876, -20.533862, -38.515158, 162.081354, 0.000000];
CF = CH4; % 2;
EF = [ -1.865940]; FQ = [ 2917.00, 1534.00, 1534.00, 3019.00, 3019.00, 3019.00, 1306.00, 1306.00, 1306.00];
TR = [ 298.00, 1300.00]; AH = [ -0.703029, 108.477300, -42.521570, 5.862788, 0.678565, -76.843760, 158.716300, -74.873100];
TR = [ 1300.00, 1600.00]; AH = [ 85.812170, 11.264670, -2.114146, 0.138190, -26.422210, -153.532700, 224.414300, -74.873100];
CF = CO; % 3;
EF = [ -1.314068]; FQ = [ 2170.00];
TR = [ 298.00, 1300.00]; AH = [ 25.567590, 6.096130, 4.054656, -2.671201, 0.131021, -118.008900, 227.366500, -110.527100];
TR = [ 1300.00, 1600.00]; AH = [ 35.150700, 1.300095, -0.205921, 0.013550, -3.282780, -127.837500, 231.712000, -110.527100];
CF = H2O; % 4;
EF = [ -3.034448]; FQ = [ 3657.00, 1595.00, 3756.00];
TR = [ 298.00, 500.00]; AH = [ 36.303952, -24.112320, 63.641110, -38.952400, -0.013850, -252.066060, 237.394310, -241.826400];
TR = [ 500.00, 1700.00]; AH = [ 30.092000, 6.832514, 6.793435, -2.534480, 0.082139, -250.881000, 223.396700, -241.826400];
TR = [ 1700.00, 6000.00]; AH = [ 41.964260, 8.622053, -1.499780, 0.098119, -11.157640, -272.179700, 219.780900, -241.826400];
CF = CO2; % 5;
EF = [ -4.385561]; FQ = [ 1333.00, 2349.00, 667.00, 667.00];
TR = [ 298.00, 1200.00]; AH = [ 24.997350, 55.186960, -33.691370, 7.948387, -0.136638, -403.607500, 228.243100, -393.522400];
TR = [ 1200.00, 6000.00]; AH = [ 58.166390, 2.720075, -0.492289, 0.038844, -6.447293, -425.918600, 263.612500, -393.522400];
CF = O2; % 6;
EF = [ -0.097960]; FQ = [ 1580.00];
TR = [ 100.00, 700.00]; AH = [ 31.322340, -20.235310, 57.866400, -36.506240, -0.007374, -8.903471, 246.794500, 0.000000];
TR = [ 700.00, 2000.00]; AH = [ 30.032350, 8.772972, -3.988133, 0.788313, -0.741599, -11.324680, 236.166300, 0.000000];
TR = [ 2000.00, 6000.00]; AH = [ 20.911110, 10.720710, -2.020498, 0.146449, 9.245722, 5.337651, 237.618500, 0.000000];
CF = NH3; % 7;
EF = [ -1.298171]; FQ = [ 3337.00, 950.00, 3444.00, 3444.00, 1627.00, 1627.00];
TR = [ 298.00, 1400.00]; AH = [ 19.995630, 49.771190, -15.375990, 1.921168, 0.189174, -53.306670, 203.859100, -45.898060];
CF = N2; % 8;
EF = [ -0.146214]; FQ = [ 2359.00];
TR = [ 100.00, 500.00]; AH = [ 28.986410, 1.853978, -9.647459, 16.635370, 0.000117, -8.671914, 226.416800, 0.000000];
TR = [ 500.00, 2000.00]; AH = [ 19.505830, 19.887050, -8.598535, 1.369784, 0.527601, -4.935202, 212.390000, 0.000000];
CF = N2O; % 9;
EF = [ 0.590717]; FQ = [ 2224.00, 1285.00, 589.00];
TR = [ 298.00, 1400.00]; AH = [ 27.679880, 51.148980, -30.644540, 6.847911, -0.157906, 71.249340, 238.616400, 82.048240];
CF = NO2; % 10;
EF = [ 0.152695]; FQ = [ 1318.00, 1618.00, 750.00];
TR = [ 298.00, 1200.00]; AH = [ 16.108570, 75.895250, -54.387400, 14.307770, 0.239423, 26.174640, 240.538600, 33.095020];
CF = NO; % 11;
EF = [ 0.820336]; FQ = [ 1904.00];
TR = [ 298.00, 1200.00]; AH = [ 23.834910, 12.588780, -1.139011, -1.497459, 0.214194, 83.357830, 237.121900, 90.291140];
CF = NO3; % 12;
EF = []; FQ = [ 1450.00];
TR = [ 298.00, 1100.00]; AH = [ 11.223160, 166.388900, -148.445800, 47.405980, -0.176791, 61.008580, 221.767900, 71.128000];
TR = [ 1100.00, 6000.00]; AH = [ 82.274180, 0.487106, -0.098769, 0.006853, -6.264954, 29.223110, 326.252800, 71.128000];
CF = HNO2; % 13;
EF = []; FQ = [];
TR = [ 298.00, 1200.00]; AH = [ 24.899740, 91.375630, -64.846140, 17.920070, -0.134737, -88.135960, 254.267100, -76.734980];
CF = HNO3; % 14;
EF = []; FQ = [];
TR = [ 298.00, 1200.00]; AH = [ 19.632290, 153.959900, -115.837800, 32.879550, -0.249114, -146.881800, 247.704900, -134.306000];
CF = HCN; % 15;
EF = [ 0.948927]; FQ = [ 3311.00, 712.00, 712.00, 2097.00];
TR = [ 298.00, 1200.00]; AH = [ 32.693730, 22.592050, -4.369142, -0.407697, -0.282399, 123.481100, 233.259700, 135.143200];
CF = C2H4; % 16;
EF = [ -0.705013]; FQ = [ 3026.00, 1623.00, 1342.00, 1023.00, 3103.00, 1236.00, 949.00, 943.00, 3106.00, 826.00, 2989.00, 1444.00];
TR = [ 298.00, 1200.00]; AH = [ -6.387880, 184.401900, -112.971800, 28.495930, 0.315540, 48.173320, 163.156800, 52.466940];
CF = CH2O; % 17;
EF = [ -1.786501]; FQ = [ 2783.00, 1746.00, 1500.00, 2843.00, 1249.00, 1167.00];
TR = [ 298.00, 1500.00]; AH = [ 5.193767, 93.232490, -44.854570, 7.882279, 0.551175, -119.359100, 202.466300, -115.897200];
CF = CH3OH; % 18;
EF = [ -3.315495]; FQ = [ 3681.00, 3000.00, 2844.00, 1477.00, 1455.00, 1345.00, 1060.00, 1033.00, 2960.00, 1477.00, 1165.00, 200.00];
TR = [ 298.00, 1500.00]; AH = [ -1.084581, 153.246357, -79.530504, 16.471302, 0.522033, -205.897417, 199.189375, -201.000000];
CF = CH3CH2OH; % 19;
EF = [ -4.325727]; FQ = [ 3653.00, 2984.00, 2939.00, 2900.00, 1490.00, 1464.00, 1412.00, 1371.00, 1256.00, 1091.00, 1028.00, 888.00, 417.00, 2991.00, 2910.00, 1446.00, 1275.00, 1161.00, 812.00];
TR = [ 298.00, 1500.00]; AH = [ -4.736788, 271.961816, -169.349465, 43.738602, 0.246434, -244.628281, 203.332562, -234.800000];
CF = CH3CHO; % 20;
EF = [ -3.115742]; FQ = [ 3005.00, 2917.00, 2822.00, 1743.00, 1441.00, 1400.00, 1352.00, 1113.00, 919.00, 509.00, 2967.00, 1420.00, 867.00, 763.00, 150.00];
TR = [ 298.00, 1500.00]; AH = [ 4.803739, 185.920024, -99.108461, 20.614739, 0.277080, -174.756600, 220.002447, -166.190000];
CF = HCOOH; % 21;
EF = [ -4.736916]; FQ = [ 3570.00, 2943.00, 1770.00, 1387.00, 1229.00, 1105.00, 625.00, 1033.00, 638.00];
TR = [ 298.00, 1500.00]; AH = [ 3.802752, 153.662179, -84.640468, 16.297378, 0.277206, -385.165270, 212.969897, -379.000000];
CF = H2O2; % 22;
EF = [ -2.034197]; FQ = [ 3617.95, 1393.50, 877.93, 370.89, 3560.00, 1273.68];
TR = [ 298.00, 1500.00]; AH = [ 34.256670, 555.184450, -35.154430, 9.087440, -0.422157, -149.909800, -257.060600, -136.106400];
6 致谢
感谢国家自然科学基金委(21703067, 21622305, 21873028),国家重大研发项目(2018YFA0208602),中国博士后科学基金(2017M611471)对本项目的支持。